Your Chair conformation rotation images are available in this site. Chair conformation rotation are a topic that is being searched for and liked by netizens today. You can Download the Chair conformation rotation files here. Get all free images.
If you’re searching for chair conformation rotation pictures information connected with to the chair conformation rotation topic, you have visit the right blog. Our website frequently provides you with hints for downloading the highest quality video and picture content, please kindly hunt and find more informative video content and graphics that fit your interests.
Chair Conformation Rotation. The chair form of cyclohexane shown on the left is the most stable conformation. The second conformation is three pairs of eclipsing groups. In total 124 16 kJmol. Constituents of the ring that project above or below the plane of the ring are axial and those that project parallel to the plane are equatorial.
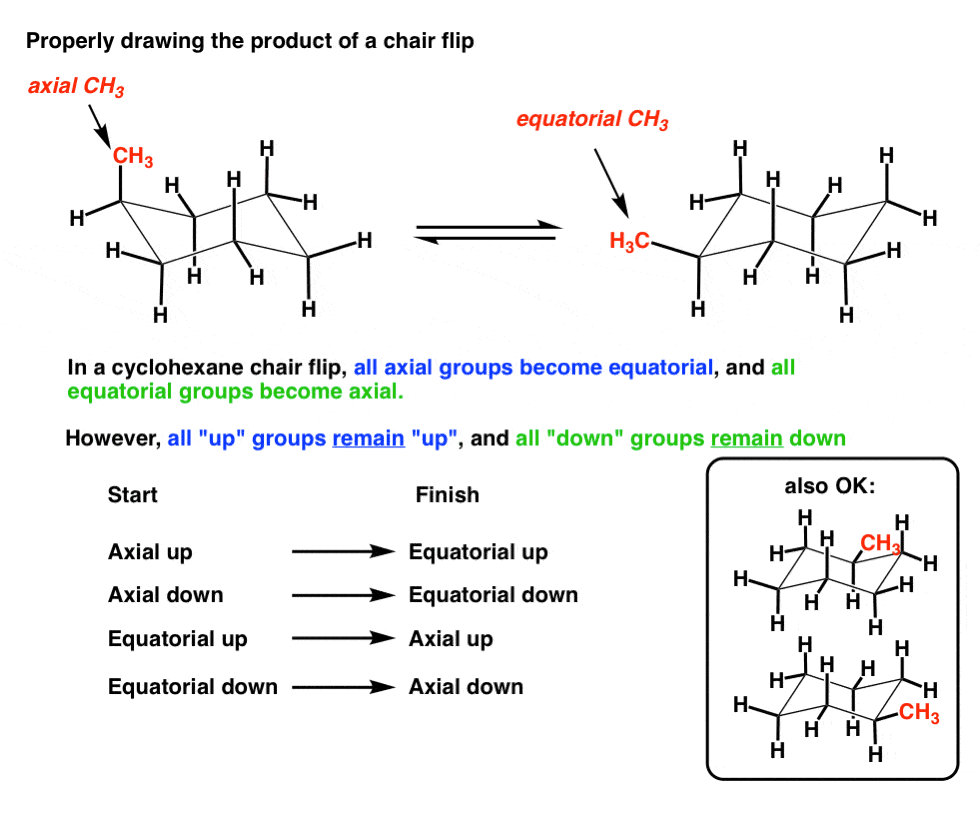 The Cyclohexane Chair Flip Master Organic Chemistry From masterorganicchemistry.com
The Cyclohexane Chair Flip Master Organic Chemistry From masterorganicchemistry.com
One HH 4 kJmol and two HCH 3 2 x 6 kJmol 12 kJmol. By twisting the boat conformation the steric hindrance can be partially relieved but the twist-boat conformer still retains some of the strains that characterize the boat conformer. This rotation from state 1 to 2 matches the 144 rotation 36 4 expected from a four-tenths rotation of the c-ring and would be expected to. Its called the S N 2 reaction and its going to be extremely useful for us going forward. Cyclohexane rapidly interconverts between two stable chair conformations because of the. The second Fischer projection below is simply a different conformation of the same sugar as the first one.
This model is a unique and high-quality heavy-duty reclining chair featuring faux.
In stereochemistry stereoisomerism or spatial isomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms constitution but differ in the three-dimensional orientations of their atoms in space. Carbohydrate conformation which includes cyclohexane conformations as well as other details. John DAngelo Michael B. The first thing you need to know before drawing the ring-flip of a chair cyclohexane is the correct conformation of the carbon-chain and the orientation of each axial and equatorial group. This is the predominant. Finally by lifting one carbon above the ring plane and the other below the plane a relatively strain-free chair conformer is formed.
 Source: chem.libretexts.org
Source: chem.libretexts.org
This contrasts with structural isomers which share the same molecular formula but the bond connections or their order. In this conformation the dihedral angle is 0. This contrasts with structural isomers which share the same molecular formula but the bond connections or their order. Disconnection of a bond away from the stereogenic center usually leads to a less efficient and less desirable. Finally by lifting one carbon above the ring plane and the other below the plane a relatively strain-free chair conformer is formed.
 Source: studocu.com
Source: studocu.com
The chair conformation is more stable than the boat conformation. This is the predominant. Free rotation around carbon-carbon single bonds allows molecules to exist in a variety of forms and different arrangements called conformations. Cycloalkane conformations including medium rings and macrocycles. Segment the others through the seams and two mirror planes including the first rotation axis.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
This rotation from state 1 to 2 matches the 144 rotation 36 4 expected from a four-tenths rotation of the c-ring and would be expected to. There are different ways of drawing a chair conformation and you are free to choose the one you like as long as at the end you have the structures correct. The difference between the energies of the chair conformation in which the hydrogen atoms are staggered and the boat conformation in which they are eclipsed is about 30 kJmol. This chapter illustrates two key points. C Skew or Gauche conformation All other conformations in between eclipsed and staggered conformations are called skew or Gauche conformations.
 Source: pinterest.com
Source: pinterest.com
The second conformation is three pairs of eclipsing groups. The second Fischer projection has the proper conformation for cyclic hemiacetal formation. Nevertheless the chair conformation is. Free rotation around carbon-carbon single bonds allows molecules to exist in a variety of forms and different arrangements called conformations. Functional groups are specific groupings of atoms within molecules that have their own characteristic properties regardless of the other atoms present in a molecule.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The key difference between axial and equatorial position is that axial bonds are vertical while equatorial bonds are horizontal. Disconnection of a bond away from the stereogenic center usually leads to a less efficient and less desirable. In stereochemistry stereoisomerism or spatial isomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms constitution but differ in the three-dimensional orientations of their atoms in space. Stereochemistry is an important issue in any synthesis. One HH 4 kJmol and two HCH 3 2 x 6 kJmol 12 kJmol.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Nevertheless the chair conformation is. Continued rotation leads to a staggered conformation in which the methyl groups are close to each other called the gauche conformation. Rotation of the bond in the anti conformation 60 o leads to an eclipsed conformation. C Skew or Gauche conformation All other conformations in between eclipsed and staggered conformations are called skew or Gauche conformations. This reclining chair was designed and produced with the health of the spine and body conformation in mind to encourage and promote full blood circulation.
 Source: pinterest.com
Source: pinterest.com
In the chair conformation the orientation of the hydroxyl group about the anomeric carbon of α-D-glucose is axial and equatorial in β-D-glucose. In a typical sophomore organic chemistry course theres about 14 functional groups that are key with another group. This is the predominant. Two reflection planes σ xz and σ yz. The first thing you need to know before drawing the ring-flip of a chair cyclohexane is the correct conformation of the carbon-chain and the orientation of each axial and equatorial group.
 Source: youtube.com
Source: youtube.com
By twisting the boat conformation the steric hindrance can be partially relieved but the twist-boat conformer still retains some of the strains that characterize the boat conformer. The difference between the energies of the chair conformation in which the hydrogen atoms are staggered and the boat conformation in which they are eclipsed is about 30 kJmol. The increase in electron-electron repulsion upon rotation from staggered to an eclipsed conformation is referred to as torsional strain. Its called the S N 2 reaction and its going to be extremely useful for us going forward. Finally by lifting one carbon above the ring plane and the other below the plane a relatively strain-free chair conformer is formed.
 Source: pinterest.com
Source: pinterest.com
Finally by lifting one carbon atom above the ring plane and the other below the plane a relatively strain-free chair conformer is formed. The SN2 Reaction Mechanism. The first thing you need to know before drawing the ring-flip of a chair cyclohexane is the correct conformation of the carbon-chain and the orientation of each axial and equatorial group. First disconnection should be done at a C C bond where one of the carbon atoms is a stereogenic center. The twist boat conformation of cyclohexane is optically active as it does not have any plane of symmetry.
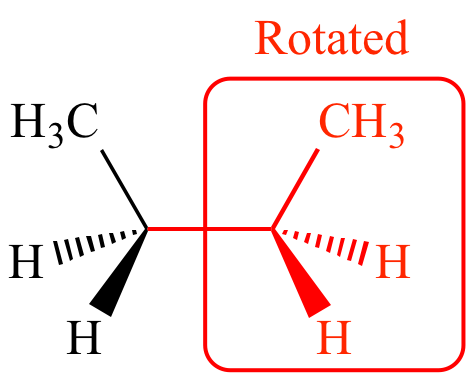 Source: chem.ucla.edu
Source: chem.ucla.edu
Carbohydrate conformation which includes cyclohexane conformations as well as other details. The terms axial and equatorial are important in showing the actual 3D positioning of the chemical bonds in a chair conformation cyclohexane molecule. By twisting the boat conformation the steric hindrance can be partially relieved but the twist-boat conformer still retains some of the strains that characterize the boat conformer. Smith in Hybrid Retrosynthesis 2015. Cyclohexane in the chair conformation has a C3 axis perpendicular to the average plane of the ring three perpendicular C2 axes between the carbons and three v planes each including the C3 axis and one of the C2 axes.
 Source: pinterest.com
Source: pinterest.com
Cyclohexane rapidly interconverts between two stable chair conformations because of the. Constituents of the ring that project above or below the plane of the ring are axial and those that project parallel to the plane are equatorial. The difference between the energies of the chair conformation in which the hydrogen atoms are staggered and the boat conformation in which they are eclipsed is about 30 kJmol. The chair form of cyclohexane shown on the left is the most stable conformation. A conformation is a shape a molecule can take due to the rotation around one or.
 Source: chemistrysteps.com
Source: chemistrysteps.com
And one 2-fold rotation axis C 2. If 0228 g of silver salt of dibasic acid gave a residue of 0162 g of silver on ignition then molecular weight of the acid is. The twist boat conformation of cyclohexane is optically active as it does not have any plane of symmetry. Cyclohexane conformations including with chair and boat conformations among others. Is a Howard Hughes Medical.
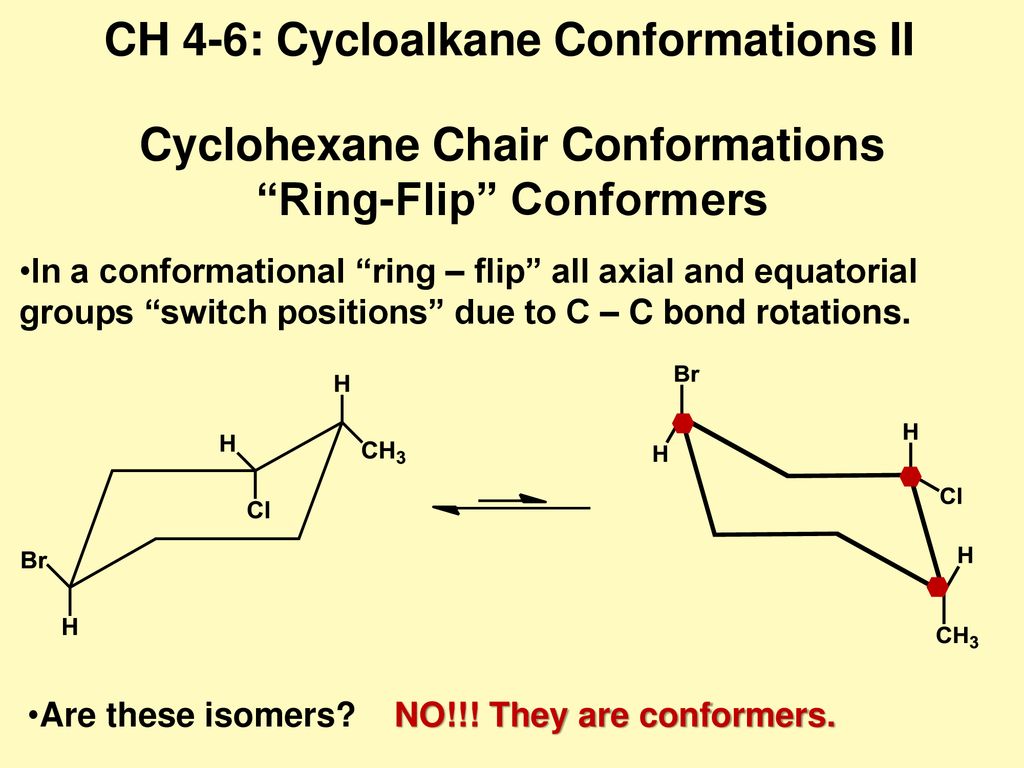 Source: slideplayer.com
Source: slideplayer.com
In this conformation the dihedral angle is 0. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. Nevertheless the chair conformation is. The rotation of the rotarod was accelerated from. Disconnection of a bond away from the stereogenic center usually leads to a less efficient and less desirable.
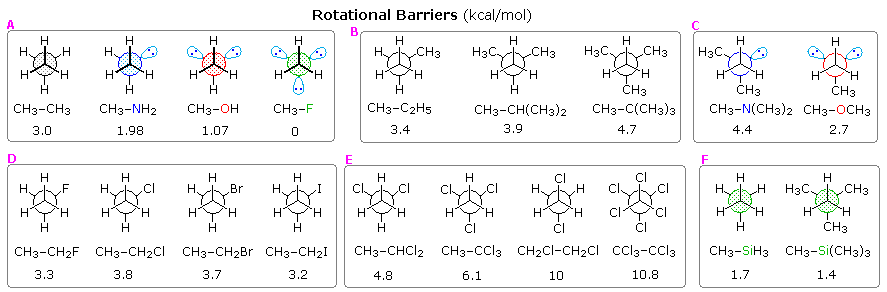 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
This chapter illustrates two key points. The key difference between axial and equatorial position is that axial bonds are vertical while equatorial bonds are horizontal. This rotation from state 1 to 2 matches the 144 rotation 36 4 expected from a four-tenths rotation of the c-ring and would be expected to. In a typical sophomore organic chemistry course theres about 14 functional groups that are key with another group. Finally by lifting one carbon above the ring plane and the other below the plane a relatively strain-free chair conformer is formed.
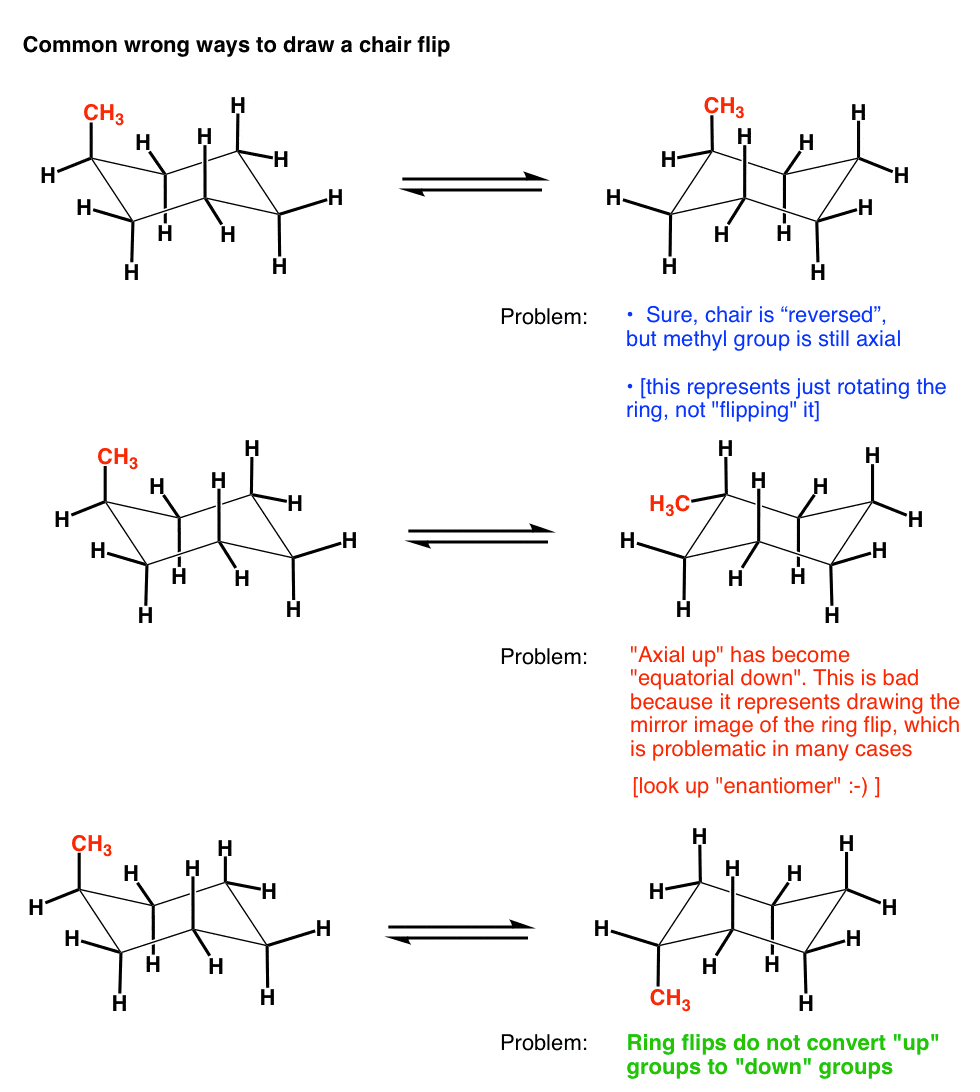 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Is a Howard Hughes Medical. Note the tip up on the left side and the tip down on the right side hence the name chair form. Disconnection of a bond away from the stereogenic center usually leads to a less efficient and less desirable. One HH 4 kJmol and two HCH 3 2 x 6 kJmol 12 kJmol. Rotation of the bond in the anti conformation 60 o leads to an eclipsed conformation.
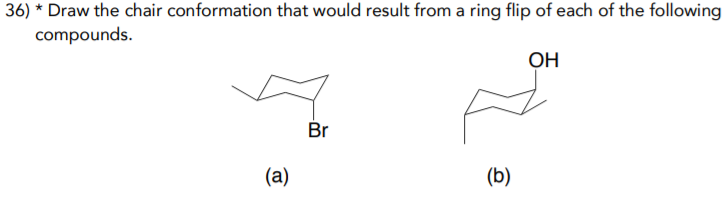 Source: chegg.com
Source: chegg.com
The difference between the energies of the chair conformation in which the hydrogen atoms are staggered and the boat conformation in which they are eclipsed is about 30 kJmol. Constituents of the ring that project above or below the plane of the ring are axial and those that project parallel to the plane are equatorial. Cyclohexane in the chair conformation has a C3 axis perpendicular to the average plane of the ring three perpendicular C2 axes between the carbons and three v planes each including the C3 axis and one of the C2 axes. Allylic strain energetics related to rotation about the single bond between an sp 2 carbon and. The chair form is the more stable of the two.
 Source: yumpu.com
Source: yumpu.com
Further rotation gives the least stable conformation in which the hydrogens are. The increase in electron-electron repulsion upon rotation from staggered to an eclipsed conformation is referred to as torsional strain. The first conformation has one CH 3 CH 3 gauche interaction which brings 38 kJmol energy of destabilization. By twisting the boat conformation the steric hindrance can be partially relieved but the twist-boat conformer still retains some of the strains that characterize the boat conformer. Continued rotation leads to a staggered conformation in which the methyl groups are close to each other called the gauche conformation.
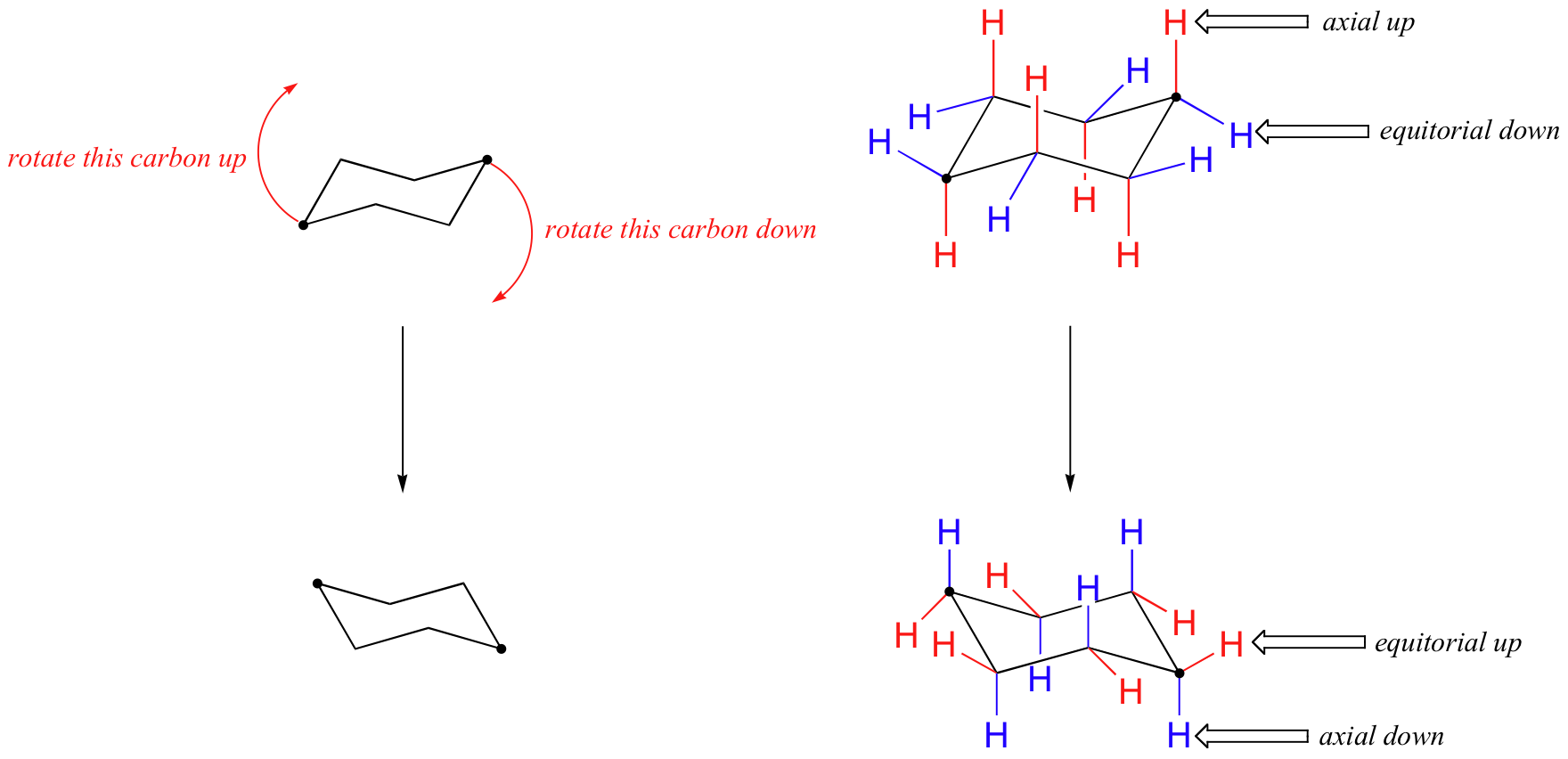 Source: chem.libretexts.org
Source: chem.libretexts.org
First disconnection should be done at a C C bond where one of the carbon atoms is a stereogenic center. The increase in electron-electron repulsion upon rotation from staggered to an eclipsed conformation is referred to as torsional strain. Common examples are alcohols amines carboxylic acids ketones and ethers. Segment the others through the seams and two mirror planes including the first rotation axis. The chair form of cyclohexane shown on the left is the most stable conformation.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title chair conformation rotation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





