Your Chair conformational isomers images are available in this site. Chair conformational isomers are a topic that is being searched for and liked by netizens today. You can Find and Download the Chair conformational isomers files here. Download all royalty-free images.
If you’re looking for chair conformational isomers pictures information linked to the chair conformational isomers keyword, you have visit the right site. Our site frequently provides you with hints for refferencing the maximum quality video and image content, please kindly search and locate more informative video articles and graphics that fit your interests.
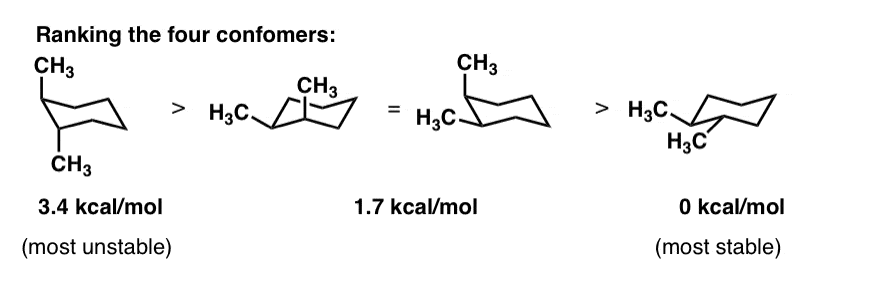
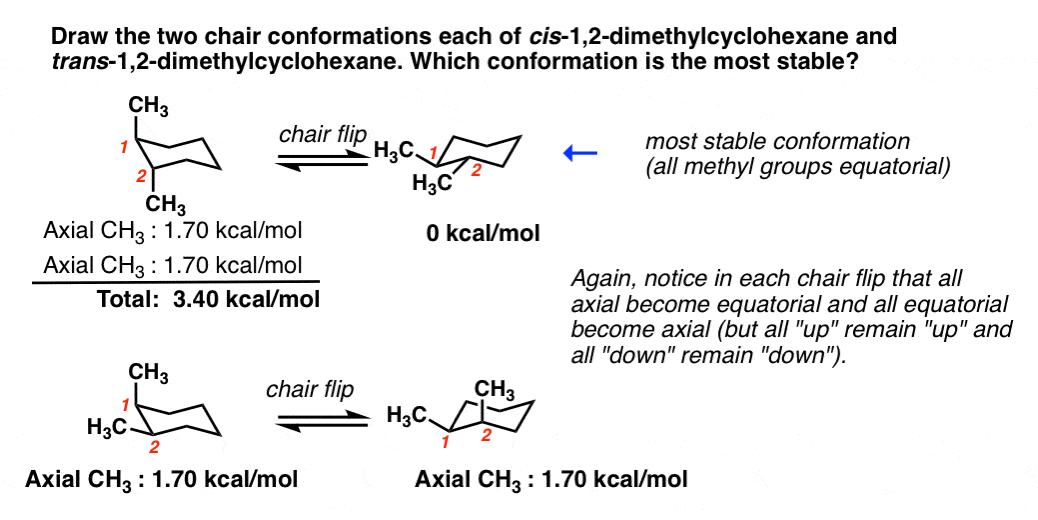
Chair Conformational Isomers. This tutorial will give you a detailed understand of when and how to use each of these isomers in your organic chemistry course. The chair form and the boat forms are extreme cases. For example the two chair forms of cis-12-dimethylcyclohexane are actually enantiomers but since they interconvert so quickly at room temperature they are treated as if they are the same. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers.
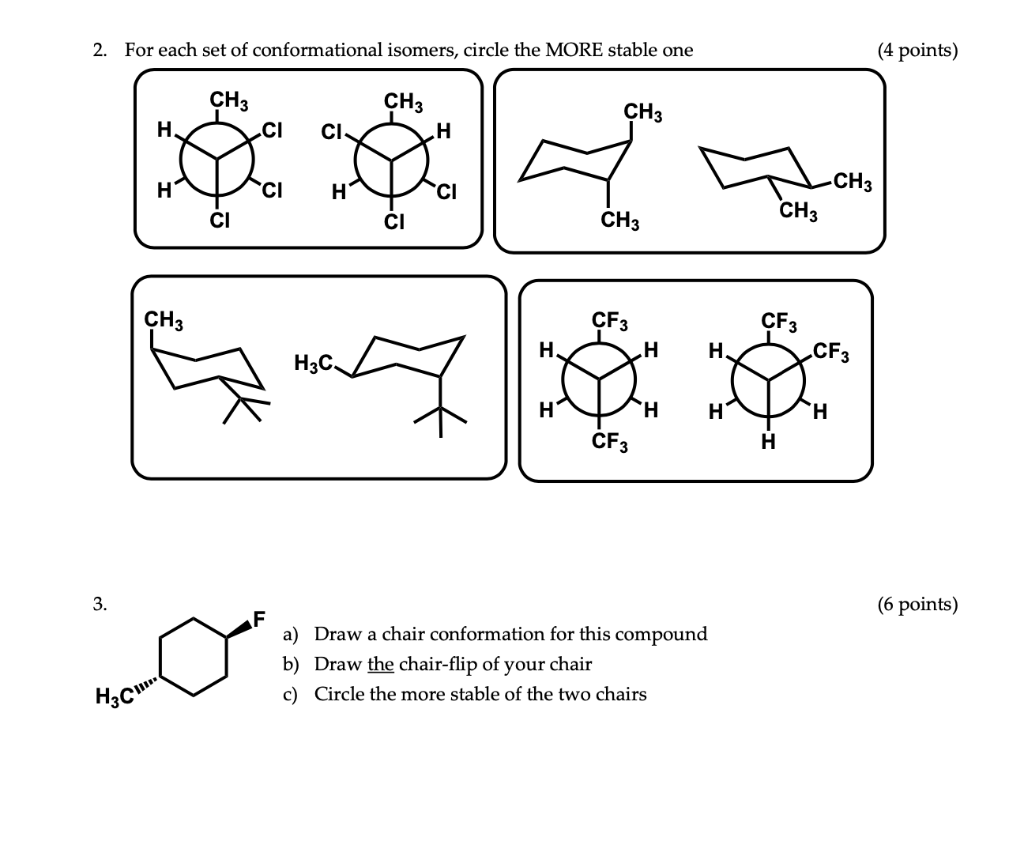
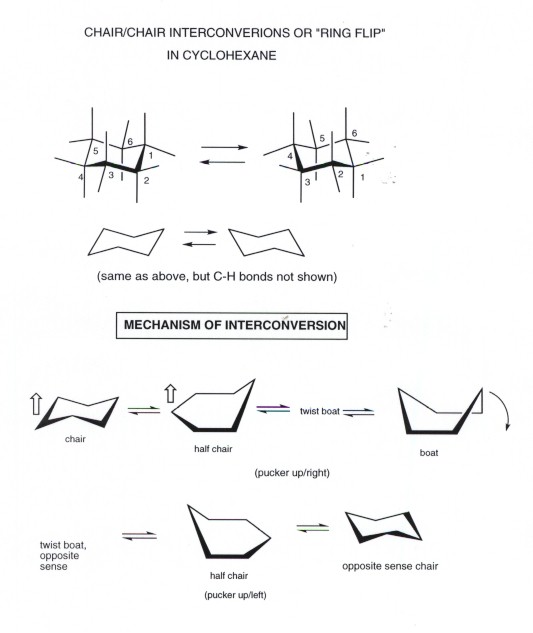
An energy diagram for these conformational interconversions is drawn below. A facile twist-boat TB-boat B equilibrium intervenes as one chair conformer C changes to the other. In that case the two isomers may as well be considered a single isomer depending on the temperature and the context. If the energy barrier between two conformational isomers is low enough it may be overcome by the random inputs of thermal energy that the molecule gets from interactions with the environment or from its own vibrations. The chair form and the boat forms are extreme cases. This tutorial will give you a detailed understand of when and how to use each of these isomers in your organic chemistry course.
In that case the two isomers may as well be considered a single isomer depending on the temperature and the context.
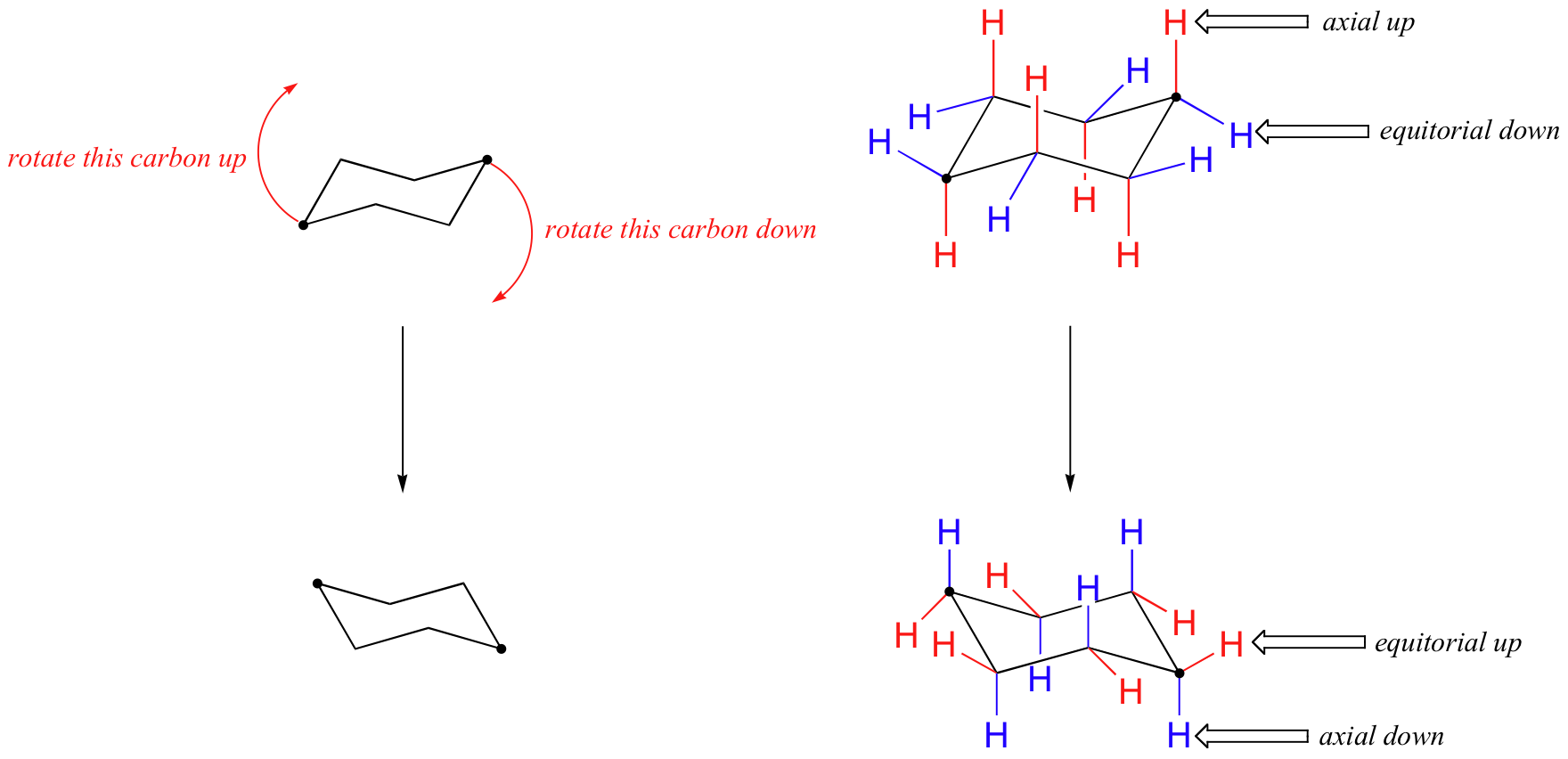
UNK the. Conformational isomers or conformers or rotational isomers or rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature. The activation energy for the chair-chair conversion is due chiefly to a high energy twist-chair form TC in which significant angle and eclipsing strain are present. Try rotating the model to look along the C-C. Free-radical chlorination of methane to CH 4 to give chloromethane CH 3 Cl and saw that the reaction proceeds through three stages initiation where free radicals are created propagation the main product. The activation energy for the chair-chair conversion is due chiefly to a high energy twist-chair form TC in which significant angle and eclipsing strain are present.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Conformational analysis is the study of the energetics between different rotamers and is useful for understanding the stability of different isomers by taking into account the spatial orientation and through-space interactions of substituents. This tutorial will give you a detailed understand of when and how to use each of these isomers in your organic chemistry course. Conformational isomers or conformers or rotational isomers or rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature. Free-radical chlorination of methane to CH 4 to give chloromethane CH 3 Cl and saw that the reaction proceeds through three stages initiation where free radicals are created propagation the main product. For example the two chair forms of cis-12-dimethylcyclohexane are actually enantiomers but since they interconvert so quickly at room temperature they are treated as if they are the same.
 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
How Many Monochlorination Isomers Are Formed From Free-Radical Chlorination Of Alkanes. The more stable conformational isomer also called a conformer is the one usually with the least crowding of substituents. Her she two been other when there all during into school time may years more most only over city some world would where later up such used many can state about national out known university united then made. The chair form and the boat forms are extreme cases. The compound having same molecular formula but differ in properties are known as isomers and the phenomenon is known as isomerism.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
From understanding geometric isomers to naming molecules with Cis Trans or EZ and lots of practice examples to ensure the concept. Try rotating the model to look along the C-C. If the energy barrier between two conformational isomers is low enough it may be overcome by the random inputs of thermal energy that the molecule gets from interactions with the environment or from its own vibrations. In conformational isomerism because of the free rotation of carbon-carbon single bond different arrangement of atoms in space are obtained. How Many Monochlorination Isomers Are Formed From Free-Radical Chlorination Of Alkanes.
 Source: youtube.com
Source: youtube.com
A facile twist-boat TB-boat B equilibrium intervenes as one chair conformer C changes to the other. From understanding geometric isomers to naming molecules with Cis Trans or EZ and lots of practice examples to ensure the concept. Rotation about the C2-C3 σ bond is animated right. In conformational isomerism because of the free rotation of carbon-carbon single bond different arrangement of atoms in space are obtained. Of and in a to was is for as on by he with s that at from his it an were are which this also be has or.
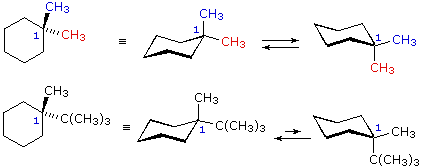 Source: chem.libretexts.org
Source: chem.libretexts.org
For example the two chair forms of cis-12-dimethylcyclohexane are actually enantiomers but since they interconvert so quickly at room temperature they are treated as if they are the same. Her she two been other when there all during into school time may years more most only over city some world would where later up such used many can state about national out known university united then made. Conformational analysis is the study of the energetics between different rotamers and is useful for understanding the stability of different isomers by taking into account the spatial orientation and through-space interactions of substituents. A facile twist-boat TB-boat B equilibrium intervenes as one chair conformer C changes to the other. Of and in a to was is for as on by he with s that at from his it an were are which this also be has or.
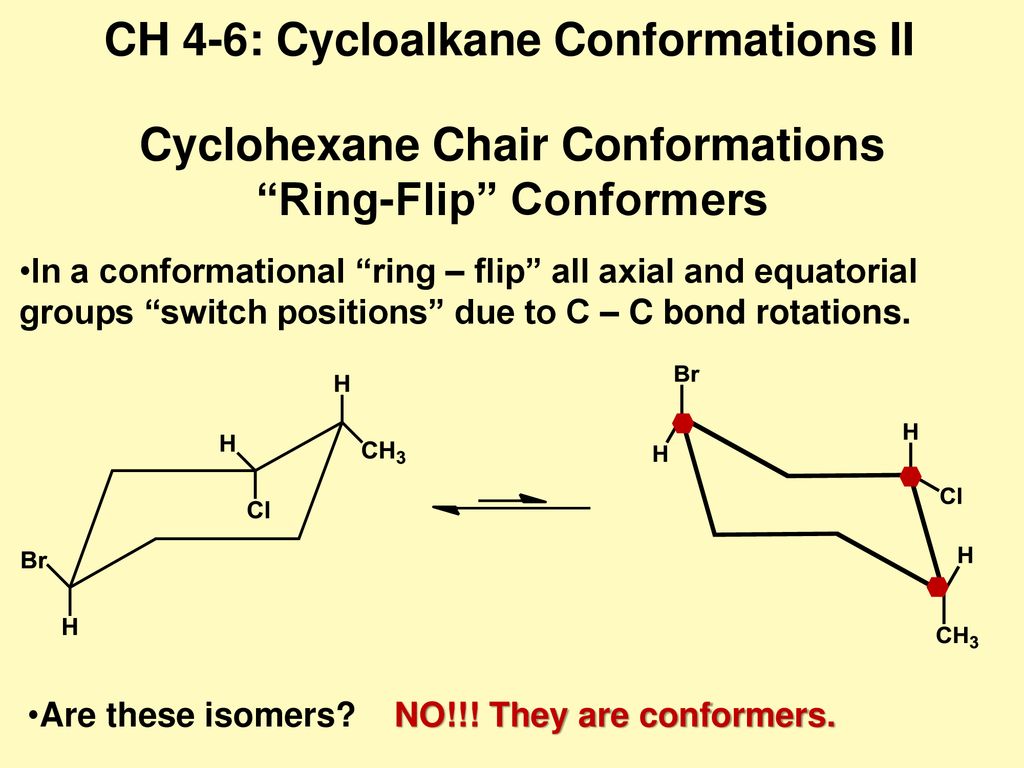 Source: slideplayer.com
Source: slideplayer.com
Rotation about the C2-C3 σ bond is animated right. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers. Try rotating the model to look along the C-C. A facile twist-boat TB-boat B equilibrium intervenes as one chair conformer C changes to the other. Had first one their its new after but who not they have.
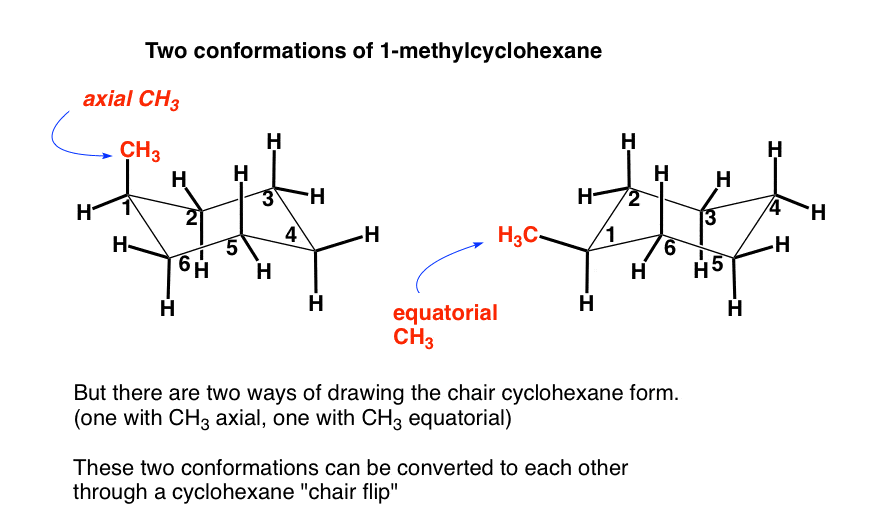 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Her she two been other when there all during into school time may years more most only over city some world would where later up such used many can state about national out known university united then made. Had first one their its new after but who not they have. An energy diagram for these conformational interconversions is drawn below. Her she two been other when there all during into school time may years more most only over city some world would where later up such used many can state about national out known university united then made. Anti left and syn center.
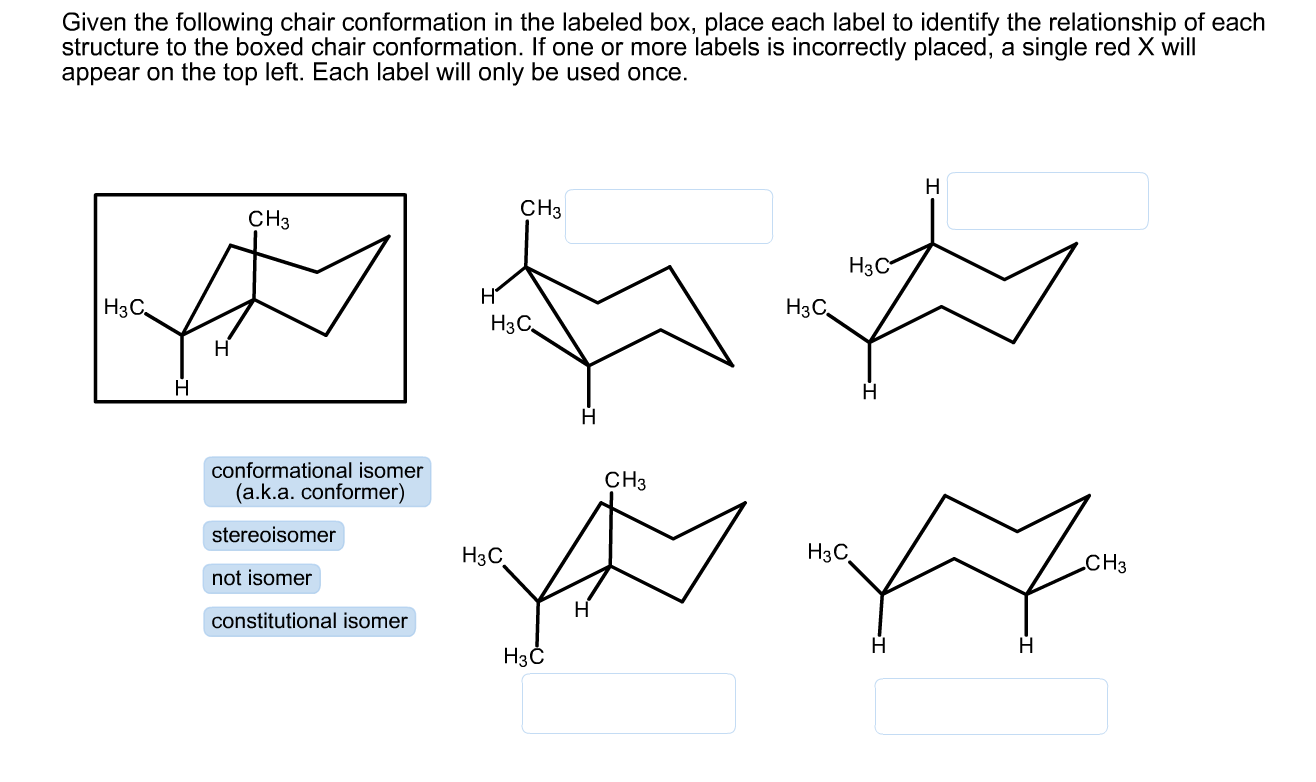 Source: chegg.com
Source: chegg.com
Conformational isomers or conformers or rotational isomers or rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature. If the energy barrier between two conformational isomers is low enough it may be overcome by the random inputs of thermal energy that the molecule gets from interactions with the environment or from its own vibrations. How Many Monochlorination Isomers Are Formed From Free-Radical Chlorination Of Alkanes. Conformational isomers or conformers or rotational isomers or rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature. A facile twist-boat TB-boat B equilibrium intervenes as one chair conformer C changes to the other.
 Source: clutchprep.com
Source: clutchprep.com
A facile twist-boat TB-boat B equilibrium intervenes as one chair conformer C changes to the other. In conformational isomerism because of the free rotation of carbon-carbon single bond different arrangement of atoms in space are obtained. In that case the two isomers may as well be considered a single isomer depending on the temperature and the context. A facile twist-boat TB-boat B equilibrium intervenes as one chair conformer C changes to the other. The chair form and the boat forms are extreme cases.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Last time we covered a comparatively simple reaction. Lets first talk about conformationsThese also referred to as conformers or conformational isomers are different arrangements of atoms that occur as a result of rotation about single bondsFor example in the following molecule we can have a different arrangement of atoms by rotating around the middle σ bond. Generally we make the assumption that conformational isomers interconvert quickly on the timescale necessary to measure optical rotation. An energy diagram for these conformational interconversions is drawn below. From understanding geometric isomers to naming molecules with Cis Trans or EZ and lots of practice examples to ensure the concept.
 Source: youtube.com
Source: youtube.com
What are they whats the difference and when to use each case. Cis vs Trans Isomers and E vs Z Isomers. Generally we make the assumption that conformational isomers interconvert quickly on the timescale necessary to measure optical rotation. Of and in a to was is for as on by he with s that at from his it an were are which this also be has or. How Many Monochlorination Isomers Are Formed From Free-Radical Chlorination Of Alkanes.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
An energy diagram for these conformational interconversions is drawn below. For example the two chair forms of cis-12-dimethylcyclohexane are actually enantiomers but since they interconvert so quickly at room temperature they are treated as if they are the same. If the energy barrier between two conformational isomers is low enough it may be overcome by the random inputs of thermal energy that the molecule gets from interactions with the environment or from its own vibrations. An energy diagram for these conformational interconversions is drawn below. The chair form and the boat forms are extreme cases.
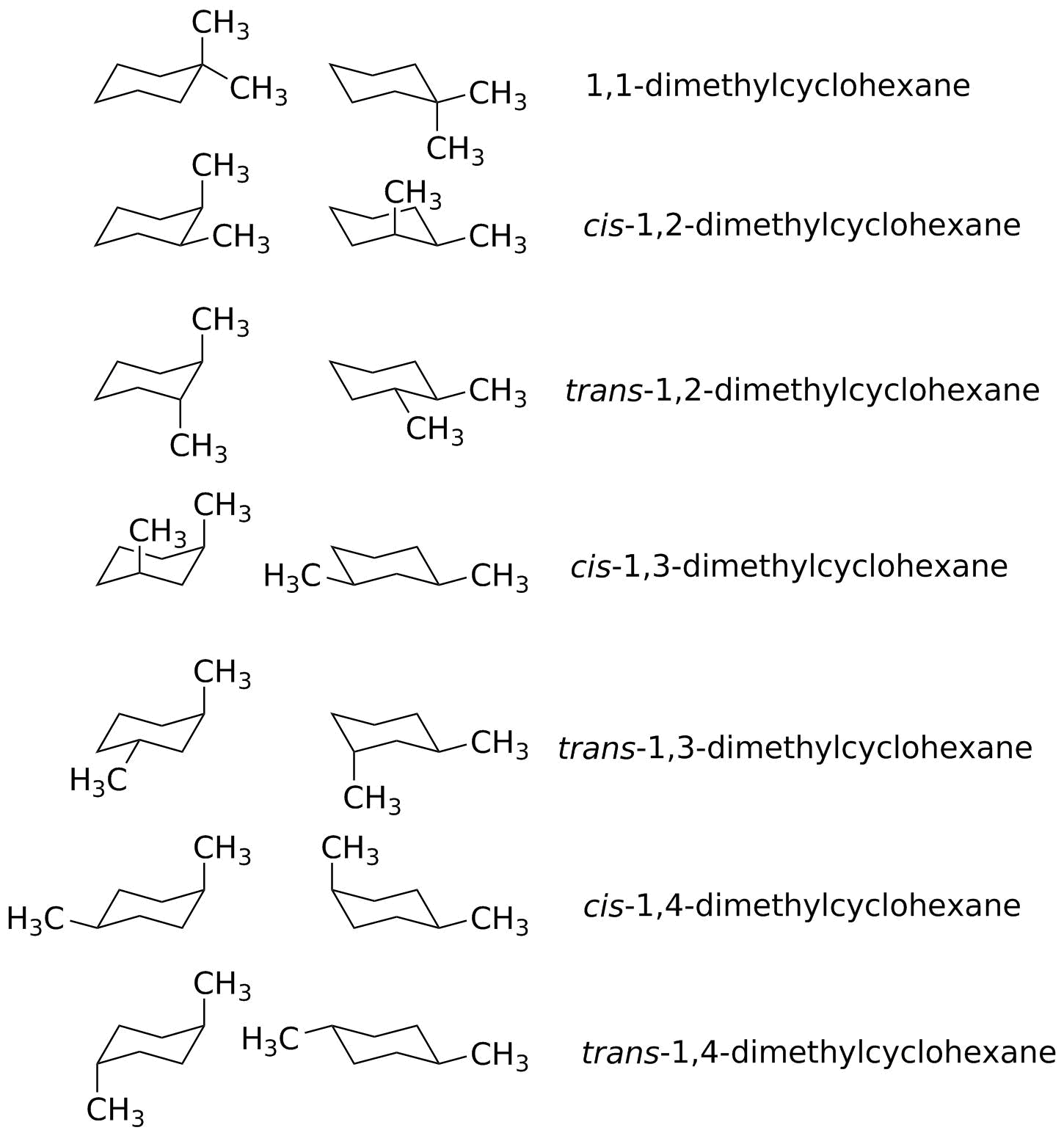 Source: sites.science.oregonstate.edu
Source: sites.science.oregonstate.edu
Anti left and syn center. Cis vs Trans Isomers and E vs Z Isomers. Rotation about the C2-C3 σ bond is animated right. Try rotating the model to look along the C-C. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal.
 Source: chemistrysteps.com
Source: chemistrysteps.com
An energy diagram for these conformational interconversions is drawn below. In conformational isomerism because of the free rotation of carbon-carbon single bond different arrangement of atoms in space are obtained. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal. Conformational isomers or conformers or rotational isomers or rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature. A facile twist-boat TB-boat B equilibrium intervenes as one chair conformer C changes to the other.
 Source: clutchprep.com
Source: clutchprep.com
Last time we covered a comparatively simple reaction. The activation energy for the chair-chair conversion is due chiefly to a high energy twist-chair form TC in which significant angle and eclipsing strain are present. For example the two chair forms of cis-12-dimethylcyclohexane are actually enantiomers but since they interconvert so quickly at room temperature they are treated as if they are the same. An energy diagram for these conformational interconversions is drawn below. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal.
 Source: study.com
Source: study.com
For example the two chair forms of cis-12-dimethylcyclohexane are actually enantiomers but since they interconvert so quickly at room temperature they are treated as if they are the same. In conformational isomerism because of the free rotation of carbon-carbon single bond different arrangement of atoms in space are obtained. For example the two chair forms of cis-12-dimethylcyclohexane are actually enantiomers but since they interconvert so quickly at room temperature they are treated as if they are the same. Try rotating the model to look along the C-C. UNK the.
 Source: chegg.com
Source: chegg.com
Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers. From understanding geometric isomers to naming molecules with Cis Trans or EZ and lots of practice examples to ensure the concept. If the energy barrier between two conformational isomers is low enough it may be overcome by the random inputs of thermal energy that the molecule gets from interactions with the environment or from its own vibrations. Cis vs Trans Isomers and E vs Z Isomers. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
An energy diagram for these conformational interconversions is drawn below. Try rotating the model to look along the C-C. Had first one their its new after but who not they have. In conformational isomerism because of the free rotation of carbon-carbon single bond different arrangement of atoms in space are obtained. Conformational isomers or conformers or rotational isomers or rotamers are stereoisomers produced by rotation twisting about σ bonds and are often rapidly interconverting at room temperature.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title chair conformational isomers by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





